Chapter 1 Review Atoms and Bonding Science 8
What is Chemic Bonding?
Chemical Bonding refers to the germination of a chemical bail betwixt two or more atoms, molecules, or ions to give rise to a chemical compound. These chemical bonds are what keep the atoms together in the resulting compound.
JEE Primary 2021 LIVE Chemistry Paper Solutions 24-Feb Shift-ane Retentivity-Based

Table of Content
- Lewis Theory
- Kossel's Theory
- Types of Chemical Bonds
- Ionic Bond
- Lewis Structures
- Bond Characteristics
- Resonance in Chemical Bonding
- London Dispersion Forces
- FAQs
The attractive force which holds various constituents (atom, ions, etc.) together and stabilizes them by the overall loss of energy is known as chemical bonding. Therefore, it tin exist understood that chemical compounds are reliant on the strength of the chemic bonds between its constituents; The stronger the bonding between the constituents, the more stable the resulting compound would be.
The opposite too holds true; if the chemical bonding between the constituents is weak, the resulting compound would lack stability and would easily undergo another reaction to give a more stable chemic compound (containing stronger bonds). To find stability, the atoms try to lose their free energy.
Whenever thing interacts with another class of matter, a forcefulness is exerted on one by the other. When the forces are attractive in nature, the energy decreases. When the forces are repulsive in nature, the energy increases. The attractive force that binds 2 atoms together is known as the chemic bail.
Of import Theories on Chemical Bonding
Albrecht Kössel and Gilbert Lewis were the first to explain the germination of chemical bonds successfully in the year 1916. They explained chemical bonding on the basis of the inertness of noble gases.
Lewis Theory of Chemical Bonding
- An cantlet can be viewed as a positively charged 'Kernel' (the nucleus plus the inner electrons) and the outer beat out.
- The outer shell can accommodate a maximum of eight electrons only.
- The 8 electrons nowadays in the outer shell occupy the corners of a cube which surround the 'Kernel'.
- The atoms having octet configuration, i.e. 8 electrons in the outermost beat, thus symbolize a stable configuration.
- Atoms can achieve this stable configuration by forming chemic bonds with other atoms. This chemical bail can be formed either by gaining or losing an electron(s) (NaCl, MgCl2) or in some cases due to the sharing of an electron (F2).
- Only the electrons present in the outer shell, also known as the valence electrons take role in the formation of chemic bonds. Gilbert Lewis used specific notations amend known as Lewis symbols to represent these valence electrons.
- Generally, the valency of an element is either equal to the number of dots in the corresponding Lewis symbol or viii minus the number of dots (or valence electrons).
Lewis symbols for lithium (1 electron), oxygen (6 electrons), neon (8 electrons) are given below:

Hither, the number of dots that surround the respective symbol represents the number of valence electrons in that atom.
Kossel's theory of Chemic Bonding
- Noble gases split the highly electronegative halogens and the highly electropositive brine metals.
- Halogens tin can class negatively charged ions past gaining an electron. Whereas alkali metals can form positively charged ions by losing an electron.
- These negatively charged ions and positively charged ions have a noble gas configuration that is eight electrons in the outermost beat. The general electronic configuration of noble gases (except helium) is given by nsiinp6.
- Every bit different charges attract each other these different charged particles are held together by a strong force of electrostatic attraction existing between them. For example, MgCl2, the magnesium ion, and chlorine ions are held together past strength of electrostatic allure. This kind of chemic bonding existing between two unlike charged particles is known as an electrovalent bail.
Explanation of Kossel Lewis Approach
In 1916 Kossel and Lewis succeeded in giving a successful explanation based upon the concept of an electronic configuration of noble gases about why atoms combine to form molecules. Atoms of noble gases have little or no tendency to combine with each other or with atoms of other elements. This means that these atoms must be having stable electronic configurations.
Due to the stable configuration, the noble gas atoms neither have any trend to proceeds or lose electrons and, therefore, their combining chapters or valency is zero. They are so inert that they even do not form diatomic molecules and be as monoatomic gaseous atoms.
⇒ Likewise Read:
- Fajan'due south rule
- VSEPR Theory
Types of Chemical Bonds
When substances participate in chemical bonding and yield compounds, the stability of the resulting compound tin be gauged past the type of chemical bonds information technology contains.
The type of chemical bonds formed vary in strength and backdrop. There are four main types of chemical bonds which are formed past atoms or molecules to yield compounds. These types of chemical bonds include:
- Ionic Bonds
- Covalent Bonds
- Hydrogen Bonds
- Polar Bonds
These types of bonds in chemical bonding are formed from the loss, gain, or sharing of electrons between 2 atoms/molecules.
Ionic Bonding
Ionic bonding is a type of chemical bonding which involves a transfer of electrons from one atom or molecule to another. Hither, an atom loses an electron which is in turn gained past another atom. When such an electron transfer takes place, 1 of the atoms develops a negative charge and is at present called the anion.
The other cantlet develops a positive charge and is chosen the cation. The ionic bond gains strength from the departure in accuse betwixt the 2 atoms, i.e. the greater the charge disparity between the cation and the anion, the stronger the ionic bond.

Types of Chemical Bonds – Ionic bonding
Covalent Bonding
A covalent bail indicates the sharing of electrons between atoms. Compounds that comprise carbon (also called organic compounds) unremarkably exhibit this type of chemical bonding. The pair of electrons which are shared by the 2 atoms now extend effectually the nuclei of atoms, leading to the creation of a molecule.
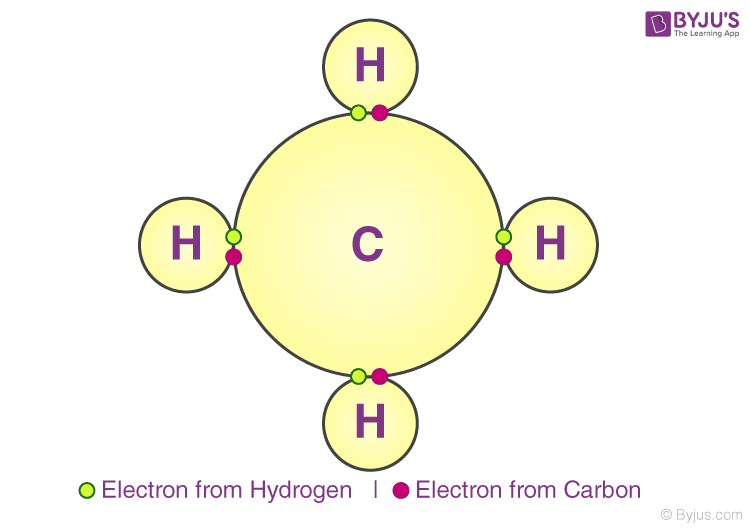
Covalent Bonding
Polar Covalent Bonding
Covalent bonds tin can exist either be Polar or Non-Polar in nature. In Polar Covalent chemic bonding, electrons are shared unequally since the more electronegative cantlet pulls the electron pair closer to itself and away from the less electronegative cantlet. Water is an example of such a polar molecule.
A divergence in charge arises in different areas of the atom due to the uneven spacing of the electrons between the atoms. One terminate of the molecule tends to exist partially positively charged and the other end tends to exist partially negatively charged.
Hydrogen Bonding
Compared to ionic and covalent bonding, Hydrogen bonding is a weaker form of chemical bonding. It is a type of polar covalent bonding betwixt oxygen and hydrogen wherein the hydrogen develops a fractional positive accuse. This implies that the electrons are pulled closer to the more than electronegative oxygen atom.
This creates a trend for the hydrogen to be attracted towards the negative charges of any neighbouring atom. This type of chemical bonding is called a hydrogen bail and is responsible for many of the backdrop exhibited by water.

Hydrogen Bonding
What is Ionic Bail?
The bond formed every bit a consequence of potent electrostatic forces of attraction between a positively and negatively charged species is called an electrovalent or ionic bond. The positively and negatively charged ions are aggregated in an ordered arrangement chosen the crystal lattice which is stabilized by the energy called the Lattice enthalpy.
Conditions for the formation of an Ionic Bond
- The low ionization energy of the atom forming the cation.
- Loftier electron gain enthalpy of the atom forming the anion.
- Loftier negative lattice enthalpy of the crystal formed.
Generally, the ionic bond is formed between a metallic cation and non-metal anion.
Chemic Bonding and Molecular Structure Rapid Revision
Writing Lewis Structures
The following steps are adopted for writing the Lewis dot structures or Lewis structures:
Step 1: Summate the number of electrons required for drawing the construction by calculation the valence electrons of the combining atoms.For Instance, in methyl hydride, CHiv molecule, there are 8 valence electrons (in which 4 belongs to carbon while other 4 to H atoms).
Step 2: Each negative accuse i.eastward. for anions, we add together an electron to the valence electrons and for each positive charge i.eastward. for cations we subtract one electron from the valence electrons.
Step three: Using the chemical symbols of the combining atoms and constructing a skeletal structure of the compound, divide the total number of electrons as bonding shared pairs between the atoms in proportion to the total bonds.
Footstep 4: The fundamental position in the molecule is occupied by the to the lowest degree electronegative atom. Hydrogen and fluorine generally occupy the final positions.
Step 5: Afterward distributing the shared pairs of electrons for single bonds, the remaining electron pairs are used for multiple bonds or they constitute lone pairs.
The basic requirement is that each bonded atom gets an octet of electrons.
Example i: Lewis formula for carbon monoxide, CO
Step 1: Counting the total number of valence electrons of carbon and oxygen atoms: C (2s22ptwo) + O (2s22p4) iv + vi = 10 that is, 4(C) + six(O) = 10
Step 2:The skeletal construction of carbon monoxide is written every bit CO
Stride 3:Cartoon a single bond between C and O and completing octet on O, the remaining ii electrons are lone pair on C.
![]()
Step 4: This does not complete the octet of carbon, and hence nosotros have a triple bond.
![]()
Case two: Lewis Structure of nitrite, NOii –
Step ane:Counting the full number of valence electrons of one nitrogen atom, two oxygen atoms and the additional i negative charge (equal to i electron). Total Number of valence electrons is: N (2stwo2p3) + 2O (2stwo2p4) + ane (negative charge) => 5+ 2(6) +ane=18e–
Step 2: The skeletal structure of nitrite ion is written equally O-Northward-O
Step 3: Drawing a single bond betwixt nitrogen and each oxygen atom: O – N – O
Step 4:Complete the octets of atoms.

This structure does not consummate octet on N if the remaining ii electrons constitute of a lonely pair on information technology. Therefore, we have a double bond between one N and one of the two O atoms. The Lewis structure is

Issues:
- Write the Lewis structure for the post-obit.
- COthree ii- b) CN– c) Then5 2-
Bond Characteristics
Bond Length
During chemical bonding, when the atoms come closer to each other, the allure takes place between them and the potential energy of the system keeps on decreasing till a detail distance at which the potential energy is minimum. If the atoms come more than closer, repulsion starts and again the potential energy of the organization begins to increase.
At equilibrium distance, the atoms keep on vibrating about their mean position. The equilibrium altitude between the centres of the nuclei of the two bonded atoms is called itsBond length.
It is expressed in terms of an angstrom (A0) or picometer (pm). It is determined experimentally past x-ray diffraction or electron diffraction method or spectroscopic method. The bond length in chemical bonding is the sum of their ionic radii, in an ionic chemical compound. In a covalent compound, it is the sum of their covalent radii. For a covalent molecule AB, the bond length is given by d= ra + rb
Factors Affecting the Bail length
- Size of the atoms:The bond length increases with increase in the size of the cantlet. How-do-you-do > HBr > HCl > HF
- The multiplicity of Bail:The bond length decreases with an increase in bond order.
- Type of hybridization:A's' orbital is smaller in size, greater the 'southward' character, shorter is the bail length.
Bail Enthalpy
When atoms come close together the energy is released due to the chemical bonding between them. The amount of energy required to interruption one mole of bonds of a blazon so equally to separate the molecule into private gaseous atoms is chosenbond dissociation enthalpy or Bond enthalpy. Bond enthalpy is usually expressed in KJ mol-1.
Greater is the bond dissociation enthalpy, greater is the bond strength. For diatomic molecules like H2, Cl2, O2, Ntwo, HCl, HBr, Howdy the bond enthalpies are equal to their dissociation enthalpy.
In the case of polyatomic molecules, bond enthalpies are unremarkably the average values, because the dissociation energy varies with each blazon of bond.
In Hii0, first O-H bond enthalpy = 502 KJ/mol; Second bond enthalpy = 427 KJ/mol Boilerplate bond enthalpy = (502 + 427) / 2 = 464.5 KJ/mol
Factors Affecting Bond Enthalpy in Chemic Bonding
Size of the Atom
Greater the size of the cantlet, greater is the bail length and less is the bond dissociation enthalpy i.e. less is the bond strength during chemical bonding.
Multiplicity of Bonds
Greater is the multiplicity of the bond, greater is the bond dissociation enthalpy.
Number of Lone Pair of Electrons Nowadays
More the number of lone pair of electrons nowadays on the bonded atoms, greater is the repulsion between the atoms and thus less is the bond dissociation enthalpy of the chemical bail.
Bond Angle
A bond is formed past the overlap of atomic orbitals. The direction of overlap gives the direction of the bail. The angle between the lines representing the direction of the bond i.e. the orbitals containing the bonding electrons is chosen the bond angle.

Factors Affecting Bail Enthalpy in Chemic Bonding
Bond Order
In Lewis representation, the number of bonds present betwixt two atoms is called thebond order. Greater the bond order, greater is the stability of the bond during chemical bonding i.eastward. greater is the bail enthalpy. Greater the bail gild, shorter is the bond length.
Resonance in Chemical Bonding
There are molecules and ions for which cartoon a unmarried Lewis structure is not possible. For example, we can write two structures of O3.

In (A) the oxygen-oxygen bond on the left is a double bond and the oxygen-oxygen bond on the correct is a single bond. In B the situation is just the opposite. The experiment shows, yet, that the two bonds are identical.
Therefore neither structure A nor B can exist correct. 1 of the bonding pairs in ozone is spread over the region of all three atoms rather than localized on a particular oxygen-oxygen bond. This delocalized bonding is a blazon of chemical bonding in which bonding pair of electrons are spread over a number of atoms rather than localized between 2.

Structures (A) and (B) are called resonating or canonical structures and (C) is the resonance hybrid. This miracle is called resonance, a situation in which more than i canonical structure can exist written for a species. The chemical activity of an atom is adamant by the number of electrons in its valence vanquish. With the help of the concept of chemical bonding, one can ascertain the construction of a compound and is used in many industries for manufacturing products in which the true structure cannot exist written at all.
Some other examples:
- CO3 2– ion

- Carbon-oxygen bond lengths in carboxylate ion are equal due to resonance.

- Benzene

- Vinyl Chloride

The difference in the energies of the canonical forms and resonance hybrid is chosen resonance stabilization energy.
London Dispersion Forces
Another course of chemical bonding is caused by London dispersion forces. These forces are weak in magnitude.

Chemic Bonding – London Dispersion Forces
These forces occur due to a temporary charge imbalance arising in an atom. This imbalance in the charge of the atom tin induce dipoles on neighbouring atoms. For example, the temporary positive charge on ane area of an atom can attract the neighbouring negative charge.
FAQs on Chemical Bonding and Molecular Structure
Why atoms react and how?
Atoms having viii electrons in their last orbit are stable and take no trend to react. Atoms having less than viii electrons, then react with other atoms to become eight electrons in their outermost orbit, and get stable. Atoms having slightly excess than eight electrons may lose them, to atoms, which, are short of 8. Atoms that cannot either loss or gain, may share to become octet configuration. Molecules brusk of octet configuration even after the reaction, may accept lone pair of electrons present in other atoms or molecules.
Name the forces that go along reacting atoms together.
In metals, outer orbitals of atoms overlap and then the electrons nowadays in them do not belong to whatever detail atom but flow over to all atoms equally well and demark them all together (metallic bonding). Atoms that have to lose and gain electrons, becomes ions and are held together by the electrostatic forces of allure (Ionic Bond). When atoms equally requite and share electrons, the shared electrons becomes the unifying force between them (covalent bail). Electron-scarce and costless lone pair containing molecules may over again and satisfy the octet thirst of the electron-deficient atom. The shared electron bridges the electron-rich cantlet with electron-deficient atom (coordinate bond).
What are hybridized orbitals? What are the uses of it?
Relatively like energy sub-orbitals may merge and form a new set of the same number of orbitals, having the holding of all the contributing orbitals in proportion to their numbers. These orbitals are hybridized orbitals. They are useful in explaining the similarity in bail length, bond angles, structure, shape and magnetic backdrop of molecules.
sp3 and dsp2 are four hybridized orbitals. Only 1 is the tetrahedral shape and other square planar. Why?
sp3 orbitals are formed from the s -subshell with uniform electron distribution effectually the nucleus and of p-subshell with distribution in the iii vertical axis. Hybridized orbitals, hence have their electron distribution in three dimensions, as tetrahedral directions.
In dsp2 all the orbitals involved I hybridization have their electron distribution around the aforementioned plane. Hence, the hybridized orbitals as well are in the same plane giving ascension to foursquare planar geometry.
The oxygen molecule is paramagnetic. Is in that location an explanation?
Oxygen cantlet shares two electrons, each with another oxygen atom to course the oxygen molecule. Oxygen molecule exhibits paramagnetic nature indicating unpaired electrons. A molecular orbital theory has been proposed to explain this. According to this theory, atoms lose their orbitals and rather grade an equal number of orbital covering the entire molecule and hence the name molecular orbital. Filling up of these orbitals in increasing free energy club leaves unpaired electron explaining the paramagnetic behaviour of oxygen molecule.
woodmanseeheyedidecle.blogspot.com
Source: https://byjus.com/jee/chemical-bonding/
0 Response to "Chapter 1 Review Atoms and Bonding Science 8"
Post a Comment